Biography
Dr. Gonzalez Bosc holds a PhD (2001) from the University of Buenos Aires (Argentina). She joined the faculty of the Cell Biology and Physiology Department in 2004. She served as director of the UNM School of Medicine Biomedical Sciences Graduate Program (BSGP) from 2020 to 2023, directed the UNM HSC Cardiovascular and Metabolic Disease Signature Program (CMVD) from 2018 to 2021, and chaired the UNM HSC Institutional Animal Care and Use Committee (IACUC) from 2014 to 2021.
Personal Statement
Members of my department have always welcomed, respected, and supported me. I practice the same values. To develop the skills and tools to promote a safe learning environment in the classroom and laboratory, I have attended workshops on upstander training, conflict resolution, and emotional intelligence. I'm committed to the mission of the UNM School of Medicine, which is to advance the health of all New Mexicans by educating the next generation of health professionals and scientists and pursuing new knowledge and excellence of practice.
Areas of Specialty
Vascular Biology, Pulmonary Hypertension, Hypoxia, Inflammation, Metabolism
Achievements & Awards
Respiration Section New Investigator Award. American Physiological Society.2010.
Dalsemer Award. Biomedical Research Grant. American Lung Association.2007.
Minority Travel Award to Experimental Biology. American Physiological Society/National Institute of Diabetes and Digestive and Kidney Diseases.2007.
Scientist Development Award. American Heart Association. Research Position. 2005. Programa Ramón y Cajal. Ministerio de Educación y Ciencia. Spain. 2004.
Affiliate Postdoctoral Fellowship. American Heart Association. 2002 and 2004.
Award of the XXVII Argentinean Congress of Cardiology. Argentinean Society of Cardiology, Argentina. 2000.
Young investigator travel award. International Society of Hypertension. 2000.
Graduate Fellowship level II. University of Buenos Aires. 1998.
Young Investigator Award. Merck Sharp & Dohme. 1999.
Graduate Fellowship level I. University of Buenos Aires. 1995.
Research fellowship (For short-term research at Laboratory of Physiology, School of Sciences. University of Balearic Islands, Spain). Ibero-American Cooperation Institute, Embassy of Spain. 1996.
Key Publications
Journal Article
Lantz, Benjamin, J. Moriwaki, Mika, Oyebamiji, Olufunmilola, M. Guo, Yan, Gonzalez Bosc, Laura, 2024 Chronic hypoxia disrupts T regulatory cell phenotype contributing to the emergence of exTreg-TH17 cells Frontiers in Physiology, vol. 14 https://doi.org/10.3389/fphys.2023.1304732
Journal Article
Sheak, Joshua, Jones, David, T Lantz, Benjamin, J Maston, Levi, D Vigil, Danielle, Resta, Thomas, C. Resta, Micaela, M Howard, Tamara, A Kanagy, Nancy, Guo, Yan, Jankowska-Gan, Ewa, Sullivan, Jeremey, A Braun, Rudolf, K Burlingham, William, J. Gonzalez Bosc, Laura, 2020 NFATc3 Regulation of Collagen V Expression Contributes to Cellular Immunity to Collagen Type V and Hypoxic Pulmonary Hypertension American Journal of Physiology-Lung Cellular and Molecular Physiology, vol. 319, Issue 6, L968--80 https://journals.physiology.org/doi/10.1152/ajplung.00184.2020
Journal Article
Maston, Levi, D. Jones, David, T. Giermakowska, Wieslawa, Howard, Tamara, A. Cannon, Judy, Wang, Wei, Wei, Yongyi, Xuan, Weimin, Resta, Thomas, C. Gonzalez Bosc, Laura, 2017 Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension American Journal of Physiology. Lung Cellular and Molecular Physiology, vol. 312, Issue 5, L609--L624
Journal Article
Maston, Levi, D. Jones, David, T. Giermakowska, Wieslawa, Resta, Thomas, C. Ramiro-Diaz, Juan, Howard, Tamara, A. Jernigan, Nikki, Herbert, Lindsay, Maurice, Anna, A. Gonzalez Bosc, Laura, 2018 Interleukin-6 trans-signaling contributes to chronic hypoxia-induced pulmonary hypertension Pulmonary Circulation, vol. 8, Issue 3, 2045894018780734
Gender
Female
Languages
- Spanish
- English
Research
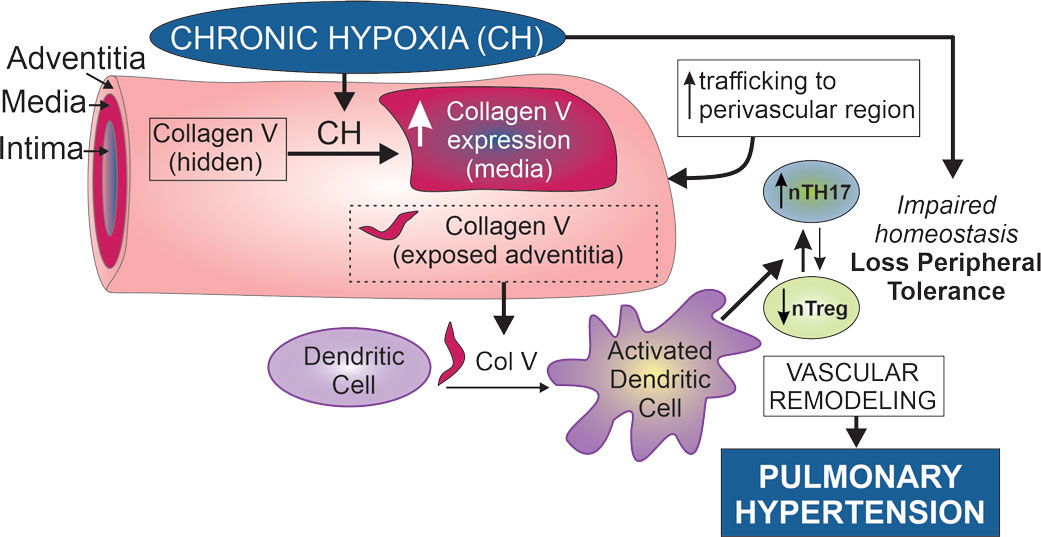
For the past 12 years, our research has focused on understanding the mechanisms that underlie the development of pulmonary hypertension caused by chronic hypoxia (CH). We have made significant contributions to this field. My group was the first one to demonstrate that the calcium-regulated transcription factor, nuclear factor of activated T-cells isoform c3 (NFATc3) is required for CH-induced pulmonary arterial remodeling and hypertension. During the last 6 years, we have been studying the role of inflammation in CH-induced pulmonary hypertension. We have established that Th17 cells are localized in the perivascular region of pulmonary arteries after CH exposure and that these cells significantly contribute to the development of pulmonary hypertension. We recently identified that CH exposes collagen V in the perivascular region of pulmonary arteries, serving as a self-antigen that triggers a Th17-mediated inflammatory response contributing to pulmonary hypertension. Our research is funded by American Heart Association, NIH and Department of Defense.
Courses Taught
Undergraduate Medical School
Phase I. Clinical Reasoning. Group Facilitator. Spring and Fall semesters. 2024 - present
Phase I. Pharmacology COPD and Asthma. Peer Instruction. Cardiovascular/Pulmonary/Renal Block. Spring 2020-2021
Cardiovascular/Pulmonary/Renal Block co-Chair 2017-2020
Phase I. Clinical Reasoning. Group Facilitator. Spring semester. 2016 - 2019
Phase I. Gastrointestinal/Nutrition Block. Tutor. 2015.
Phase I. Cardiovascular/Pulmonary/Renal Block. Tutor. 2014.
Phase I. Cardiovascular/Pulmonary/Renal Block. Tutor. 2014.
Biomedical Sciences Program
Course Director & Instructor, Biomed 501. University of New Mexico Health Science Center. Spring 2020 - 2023.
Course Director, Graduate Physiology (Biomed. Sci. 510). University of New Mexico Health Science Center. Spring 2010, 2015 and 2016.
Graduate Physiology (Biomed. Sci. 510). University of New Mexico Health Science Center. Lecturer (8 lecture hours/year). 2005 - Present.
Advanced Topics in Cellular and Systems Physiology (Biomed. Sci. 657). University of New Mexico Health Science Center. Lecturer (15 contact hours/year). 2005, 2007, 2011, 2013, 2017, 2020.
Research and Scholarship
Hypoxia results from chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD), sleep apnea, or residing at high altitude, and, through yet-undefined mechanisms, leads to a myriad of pathophysiological responses, including systemic inflammation, skeletal muscle dysfunction, and pulmonary hypertension. The scientific premise of our research is that hypoxia-induced immunity against self contributes to hypoxia-induced pulmonary vascular dysfunction. Our lab aims to address the mechanisms underlying this phenomenon to design novel therapeutic strategies to treat conditions associated with chronic hypoxia.
Advancements we made in understanding chronic inflammation in hypoxic pulmonary hypertension
Our laboratory has been exploring the role of chronic inflammation in chronic hypoxia (CH)-induced pulmonary hypertension pathogenesis. We have recently established that TH17 cells infiltrate the perivascular region of pulmonary arteries after exposure to CH and that these cells significantly contribute to the pathogenesis of pulmonary hypertension. We demonstrated that IL-6 trans-signaling contributes to this process. A collaboration with Dr. William Burlingham's research group from the University of Wisconsin at Madison set my group on the course to study the role of collagen V in CH-induced lung inflammation and pulmonary hypertension. As a result, we discovered that the expression of the transcription factor NFATc3 in smooth muscle significantly contributes to CH-induced pulmonary hypertension, partly by regulating the expression of the self-antigen COL5A1, contributing to col V-reactive TH17-mediated inflammation and hypertension. We have also demonstrated that CH causes a reduction in active T regulatory cells (Tregs) and promotes T regs with less suppressive capacity, called exTregs, that express markers of TH17 cells. These exTregs-TH17 are pathogenic, and we are currently studying whether they recognize collagen V and develop strategies to diminish collagen V reactivity to attenuate CH-induced pulmonary hypertension.
Recent publications from the lab:
- Benjamin J. Lantz, Mika Moriwaki, Olufunmilola M. Oyebamiji, Yan Guo, Laura Gonzalez Bosc. Chronic hypoxia disrupts T regulatory cell phenotype contributing to the emergence of exTreg-TH17 cells. Front. Physiol., 29 January 2024.Sec. Cell Physiology Volume 14 - 2023, doi.org/10.3389/fphys.2023.1304732. PMCI10859758.
- Joshua R. Sheak, David T. Jones, Benjamin J Lantz, Levi D. Maston, Danielle Vigil, Thomas C. Resta, Micaela M. Resta, Tamara A. Howard, Nancy L. Kanagy, Yan Guo, Ewa Jankowska-Gan, Jeremy A. Sullivan, Rudolf K. Braun, William J. Burlingham, and Laura V. Gonzalez Bosc. NFATc3 Regulation of Collagen V Expression Contributes to Cellular Immunity to Collagen Type V and Hypoxic Pulmonary Hypertension. Am J Physiol Lung Cell Mol Physiol. 2020. 319(6):L968-L980. PMC7792682
- Maston LD, Jones DT, Giermakowska W, Resta TC, Ramiro-Diaz J, Howard TA, Jernigan NL, Herbert L, Maurice AA, Gonzalez Bosc LV. Interleukin-6 trans-signaling contributes to chronic hypoxia-induced pulmonary hypertension. Pulm Circ. 2018 Jul-Sep;8(3):2045894018780734. PMC6055240
- Levi D. Maston, David T. Jones, Wieslawa Giermakowska, Tamara A. Howard, Judy L. Cannon, Wei Wang, Yongyi Wei, Weimin Xuan, Thomas C. Resta, Laura V. Gonzalez Bosc. Central Role of T Helper 17 Cells in Chronic Hypoxia-Induced Pulmonary Hypertension. Am J Physiol Lung Cell Mol Physiol. 2017. 312(5):L609-L624. PMC5451600

