The Brigman lab studies how neurodevelopmental insult and neuropsychiatric disease leads to maladaptive behavioral changes that decrease quality of life with the goal of rescuing these deficits and improving outcomes.
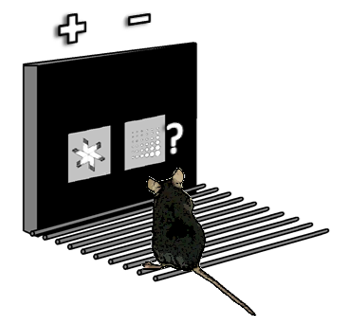
Traditionally, studies in rodents utilize behaviors that focus on their strengths (digging, smelling, navigating). While these approaches have yielded a wealth of data, they differ greatly from how we measure cognitive function n humans. A major goal of the lab is to develop and validate new assays for investigating behavioral outcomes that can be more directly compared to clinical data. Beginning in 2001 I have worked to develop and refine tasks utilizing touchscreen approaches to screen learning, memory and executive control behaviors. This work helped establish the utility of the mouse in executive function research and led to the wider adoption of touchscreen operant paradigms for high-throughput screening. For the past 10 years my lab has integrated in vivo recording of the activity of cortical neurons and local field potentials during these tasks in vivo to help us better understand how different brain regions mediate specific behaviors. More recently, we have been involved in a multi-university study to compare neural activity associated with specific behavior in both rodents and humans to test the utility of rodent behavioral data in therapeutic target development.
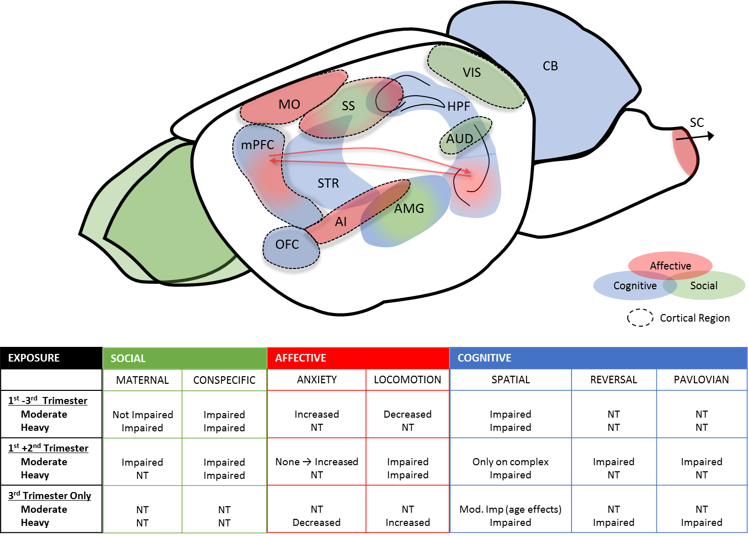
The impact of alcohol on cortical function and the executive control processes it underlies is a critical issue as loss of these key processes can lead to a major decline in quality of life. Although the impact of developmental or adult exposure on spatial, and to a lesser extent operant, tasks has been well documented, few studies have used online measurement of neuronal function to parse circuit level alterations after exposure. Utilizing techniques in vivo recording techniques, The Brigman lab even a more moderate prenatal exposure is sufficient to impair behavioral flexibility and alter cortical firing and recruitment. In addition, PAE dysregulated cortico-striatal coordination required for behavioral flexibility. Recently, we have shown that PAE also alter reinstatement behavior and visuo-spatial discrimination in otherwise EtOH-abstinent into early adulthood. Excitingly, we have also shown that early life environment can significantly alter outcomes in these models.
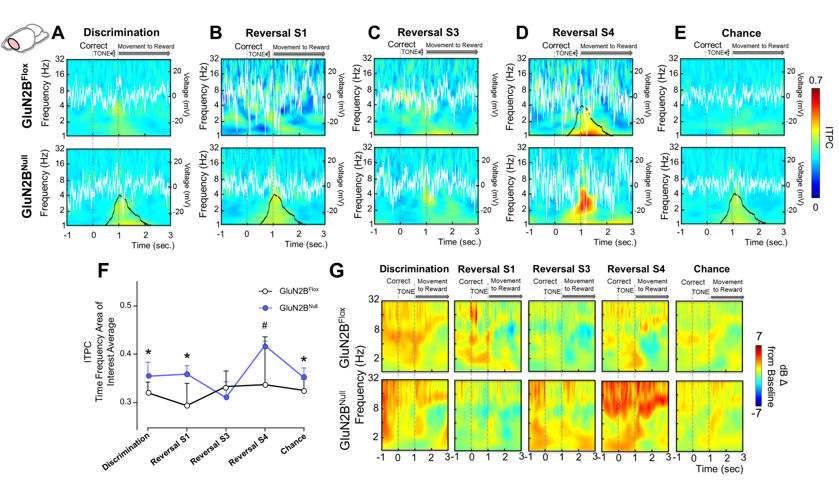
Loss of specific NMDAR subtypes in the cortex, hippocampus and striatum can alter both learning and synaptic plasticity. Based on foundational studies conducted in the Holmes lab, my lab has investigated the role of GluN2A and GluN2B containing NMDAR using forebrain wide and conditional knock-out models and pharmacological inactivation. We have shown that loss of GluN2A brain-wide impairs simple learning and flexibility in a set-shifting task. Further, we showed that loss of GluN2B in the cortex and hippocampus spares discrimination learning but impairs the ability to learn appropriate rules and apply those rules to novel problems. More recent studies combined GluN2B knock-out mice with in vivo recording during behavior to show that loss of the subunit alters both cortical and stratal activity, and how the regions communicate. Given findings that developmental insults like prenatal alcohol exposure alters NMDAR subunit expression, we are currently investigating whether this expression may mediate efficient learning and shifting.
Impairments in executive function are a common feature of numerous neuropsychiatric disorders. In fact, cognitive deficits may have a larger negative impact on quality of life for patients with Schizophrenia than either negative or positive symptoms. Preclinical models of Schizophrenia have shown how loss of specific systems led to behavioral impairments on translational tasks. Previously, I showed that loss of GABAergic function in the forebrain was sufficient to alter attention and impair reversal learning while chronic phencyclidine altered social investigation measures but spared learning and reversal. Recently, The Brigman Lab, in collaboration with the Mellios Lab at UNM, has shown that knockdown of circHomer1a, a neuronal-enriched circRNA expressed in the frontal cortex, was sufficient to impair reversal on our touchscreen visual discrimination task. Importantly, the Mellios laboratory also showed that circHomer1a was significantly reduced in postmortem samples of PFC tissue in patients with Schizophrenia and Bipolar Disorder. Current ongoing collaborative studies are investigating the mechanisms of how alterations in circular microRNAs may impair behavior.