
The McKenzie lab studies how memories are made, how patterns of neural activity form and propagate, and how to control the spread of seizures.
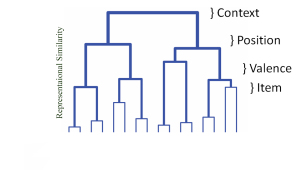
Despite being one of the most studied brain regions, the core function of the hippocampus remains unknown. My work has been guided by the relational memory framework of hippocampal function. According to this theory, events separated in time and space may be related to one another by virtue of associative linking in hippocampal circuits. This spatiotemporal bridge allows for the discovery of new rules and categories, which I see as the main function of this memory system.
Somehow, the hippocampus is able to bind together cortical regions that otherwise would have weak associativity. In the parlance of machine learning, this hippocampal-dependent coherence expands the feature space over which learning may occur. I am interested in how regularities are extracted from particulars. Specifically, my lab studies how category learning is influenced by the expanded feature space afforded by hippocampal/cortical interactions.
Over a lifetime, 1 in 26 Americans will be diagnosed with epilepsy. The pharmacological treatments that are available carry significant side effects and are not effective in 30% of the population who often suffer years before seeking alternative treatments, such as surgical resection of the seizure focus. In cases where the focus is unknown, when there are multiple foci, or when resection is too risky, deep brain stimulation may be an option. There are currently two FDA approved options: chronic stimulation of the anterior nucleus of the thalamus, and closed-loop, “responsive” stimulation to the seizure onset zone. Nobody knows why these stimulation protocols are effective, which patients may benefit the most, or the best strategy for dictating when and how to stimulate.
My lab is working towards developing seizure forecasting algorithms to detect seizures before they begin. This advanced warning will be used to perturb neural activity to desynchronize the brain and hopefully stop the spread ictal activity from the onset zone to otherwise healthy brain regions.
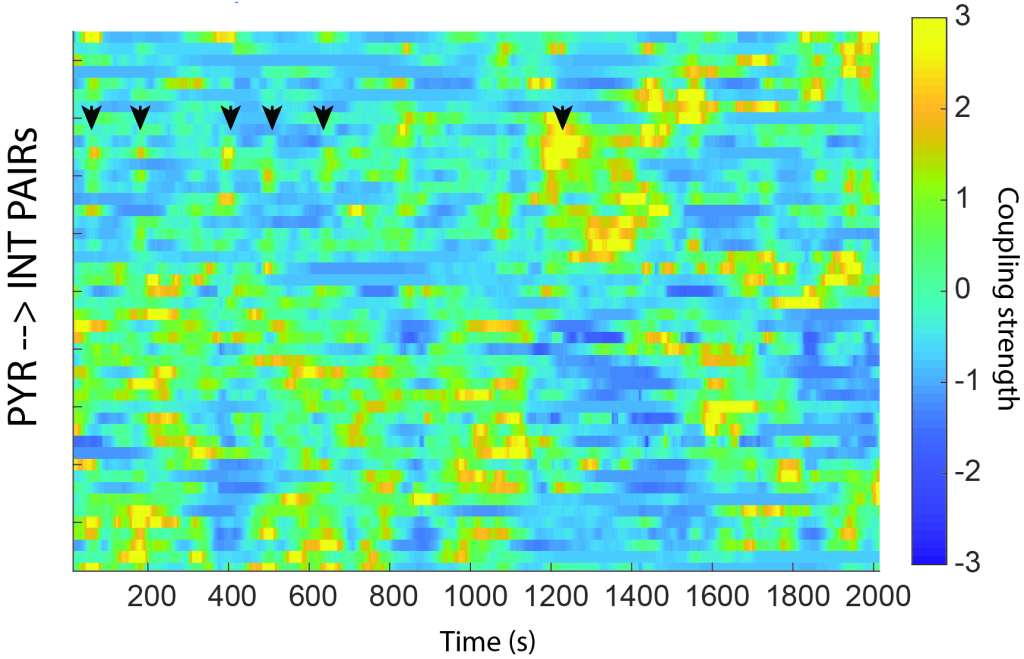
Nobody knows which feature of neural activity the brain uses to communicate with itself. It could be the identity of which neuron fires, the rate in which those neurons fire over some time window, the pattern of synchronous activity within some time window, or even the order in which neurons fire relative to one another. I think the pattern of synchronous activity matters and that learning is supported by changes in which neurons fire together in any given circumstance. My lab studies how excitatory neurons compete with one another to be active together in short time windows. I want to know whether plasticity in lateral inhibition may dictate who may fire with whom and under what circumstances those rules of coexistence apply.
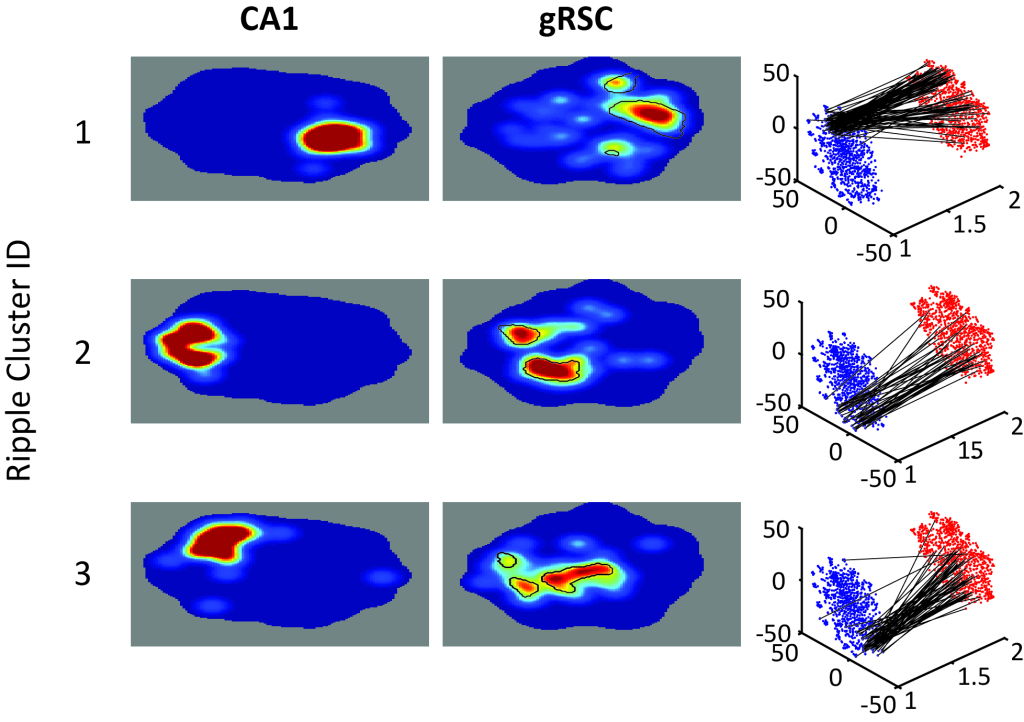
Complex correlation patterns and finely balanced synchrony only matter if those spikes can drive divergent activity in efferent brain regions. How are patterns of synchronous activity read out? How do incoming signals interact with ongoing activity that emerged through intra-region, recurrent connectivity? Can we tease apart the unique contribution of inputs from one brain region to the activity in another to figure out a synaptic transfer rule at any synapse in these central memory circuits.