An unprecedented public health crisis of prescription opioid abuse both regionally and nationally is currently gripping the United States. While the underlying causes related to this public health crisis are largely speculative, one possible contributing factor is the dramatic escalation of prescription opioid treatment for chronic pain over the past decade. Preclinical studies convincingly support that not only do opioids exert negative consequences for pain, but also that chronic pain can be targeted by non-opioid drugs that lack direct actions on neurons thereby reducing addiction liability. Trafficking immune cells to the central nervous system (CNS) and immune-like glial cells within the CNS (e.g. astrocytes and microglia) are necessary for the development and maintenance of acute-to-chronic pain problems through inappropriate neuroimmune activation. One immune receptor found in the CNS is Toll-like receptor 4 (TLR4) that is known to induce the release of proinflammatory cytokines, such as interleukin-1b (IL-1b). Non-opioid compounds that block the TLR4-IL-1b pathway may provide substantially better therapeutic benefit than opiate drugs. One goal of my laboratory is to identify non-opioid drugs that harness endogenous anti-inflammatory mechanisms resulting in the suppression of proinflammatory cytokines such as IL-1b, providing a novel approach to treat chronic pain in people while lacking potential for addictive side effects. In addition to testing several novel and FDA-approved compounds, my lab works closely with UNM’s Center for Molecular Drug Discovery http://unmcmd.health.unm.edu to identify and test potentially novel pain therapeutics that avoid opioid-related side effects.
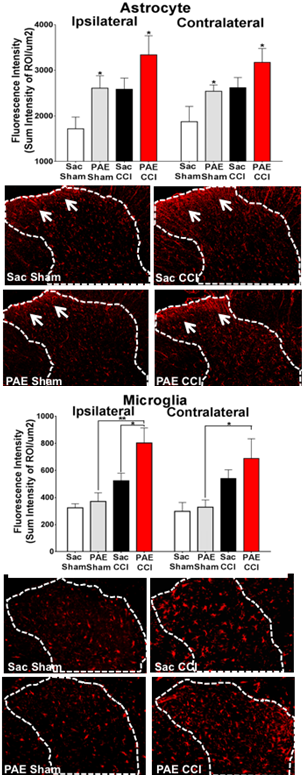
Exposure to alcohol during gestation can lead to a constellation of mild to severe disabilities that includes cognitive and behavioral deficits representing a continuum referred to as Fetal Alcohol Spectrum Disorders (FASD), with a prevalence of ~4.8% in some US regions. A growing body of evidence strongly implicates the adverse impact of alcohol exposure during central nervous system (CNS) development on cellular and molecular programing of neuroimmune function. In animal models of prenatal alcohol exposure (PAE), expression of the brain’s immune signaling molecules, the proinflammatory cytokines interleukin-1 (IL-1β), tumor necrosis factor-alpha (TNF-) and the chemokine CCL2, are significantly elevated. While evidence of sensory abnormalities including tactile sensitivity observed in children with FASD are thought to be a result of psychosocial factors, the underlying cause may include neurological dysfunction. Indeed, animal models of PAE reveal heightened sensitivity to light touch, a well-known pathological sensory condition mediated by aberrant neuronal actions in the spinal cord. Clinically, touch hypersensitivity is known as allodynia in chronic pain patients, and animal models of allodynia show pathological activation of pain neurons occurs in the spinal cord mediated by IL-1β, TNF- and CCL2. Glial cells (astrocytes & microglia) are key producers of these proinflammatory cytokines. Thus, animal models of allodynia and PAE reveal a surprising neuroimmune overlap. The goal of this research is to identify spinal cell types other than neurons that play important roles in neuroimmune adaptations in PAE offspring that enhance adult susceptibility to neuropathy. Results will provide new knowledge for understanding the developmental origins of aberrant PNS- and CNS-immune interactions due to PAE, revealing susceptibilities to adult onset diseases such as neuropathic pain.
My laboratory works closely with the New Mexico Alcohol research Center (NMARC; New Mexico Alcohol Research Center ) to understand the neurobiological mechanisms underlying FASD-related CNS disease, and to use this knowledge to develop more effective interventions for patients with FASD.
3. Mechanisms of Glucocorticoid Resistance in Prenatal Alcohol Exposure
Many of the physiological processes affected by prenatal alcohol exposure (PAE) are regulated by glucocorticoids. Glucocorticoid resistance (i.e., reduced sensitivity to the actions of GCs) and glucocorticoid receptor insensitivity is associated with a variety of chronic diseases, many of which are immune function related as well as associated with PAE. The research focus is to address whether PAE produces glucocorticoid receptor insensitivity that is expressed as dysregulation of the hypothalamic pituitary axis and an increase in brain proinflammatory and decrease in anti-inflammatory cytokines under stressful conditions, and whether a developmental shift in the normal stress and immune responses during early life is observable, and if these changes persist well into adulthood. The goal of my lab’s research is to identify molecular mechanisms underlying alterations in the programming of glucocorticoid sensitivity during CNS development that persist well into adulthood. This information will be used to develop targeted interventions that reverse or reduce the effects of PAE on glucocorticoid receptor sensitivity and responses of neuroimmune signaling molecules. To achieve these goals, my laboratory collaborates with the New Mexico Alcohol Research Center (NMARC; New Mexico Alcohol Research Center).