Bradfute Lab

Infectious diseases/pathogens as those that have newly appeared in a population or have existed but are rapidly increasing in incidence or geographic range

The deer mouse Peromyscus maniculatus carries the Sin Nombre hantavirus, which is responsible for most of the hantavirus cardiopulmonary syndrome cases in the US.
Photo credit: Sam Goodfellow

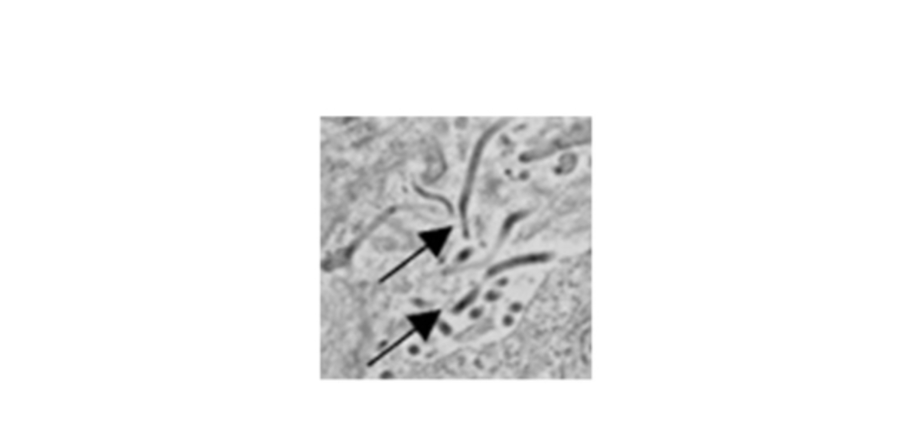
Ebola virus in infected spleen. Arrows point to individual virions. Imaging performed using transmission electron microscopy.
Photo Credit: Steven Bradfute, PhD

Zika virus plaque assay to identify neutralizing antibodies
Photo Credit: Steven Bradfute, PhD
Ebola virus infection causes death of immune cells. Fluorescence microscopy shows spleen cells from an infected animal. Red marks dying cells and shows all cells.
Photo Credit: Steven Bradfute, PhD
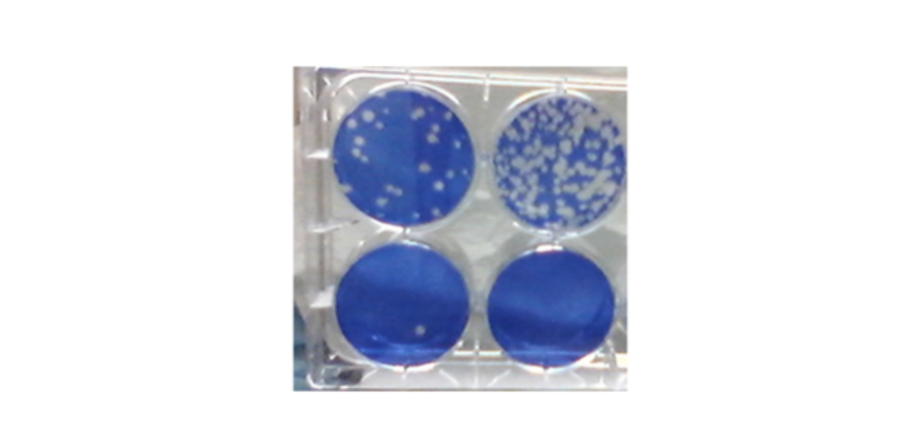
SARS-CoV-2 plaque assay to measure inhibition of coronavirus replication. Blue color is live cells; whie holes are "plaques show where SARS-CoV-2 has infected and killed cells.
Photo Credit: Steven Bradfute, PhD
Hantaviruses are dangerous pathogens that cause severe illness in humans. The viruses replicate harmlessly in rodents and are transmitted to humans via inhalation of virus in aerosolized rodent excreta. The first pathogenic hantavirus in the US, Sin Nombre hantavirus, was discovered in New Mexico in 1993. New Mexico has had the most cases of Sin Nombre hantavirus than any other state, making it an important virus to our region, especially given its high lethality rate in humans (~35%). Our research covers multiple areas of hantavirus biology and therapeutics.
-Neutralizing antibodies for therapeutics.
We have a collaborative effort as part of a NIH U19 grant based out of Albert Einstein College of Medicine to identify and analyze monoclonal antibodies against multiple hantaviruses. These data are used by the consortium to select antibodies to test for their ability to rescue small animal models from infection. This collaborative study is highlighted in “This Week in Virology” podcast: https://www.microbe.tv/twiv/twiv-578/
--Long-term memory immune responses in Sin Nombre hantavirus survivors.
We are tracking long-term immune responses in Sin Nombre hantavirus survivors. To date, we have found very strong antibody responses in survivors, with patients as far out as 23 post-infection still showing detectable neutralizing antibody responses. These data suggest hantavirus survivors generate strong, long-lasting immunity against re-infection. We have also established a 13-color flow cytometric assay to test specific hantavirus recall responses in multiple memory CD8+ and CD4+ T cell compartments.
--Genetic analysis of Sin Nombre Virus in wild-caught rodents in New Mexico.
The host rodent for Sin Nombre hantavirus is the deer mouse Peromyscus maniculatus. Deer mice have a very extensive natural range, as they are found in almost all of the US (excluding regions in the southeast). However, Sin Nombre virus infection in humans is mostly concentrated in the western and southwestern US. We are asking the question “why are the deer mice everywhere but infected humans are not?” We are answering this question by trapping wild deer mice throughout New Mexico, in both human infection-endemic and non-endemic regions, to determine if a) Sin Nombre virus is only found in human patient-endemic regions or b) the virus genome sequence is significantly different in different regions.

The Bradfute laboratory capitalized on our expertise in emerging viral pathogens to study Zika virus during the 2013-2016 outbreak. We collaborated with Los Alamos National Laboratories to test the efficacy of a novel DNA/nanoparticle delivery system in protecting mice from Zika virus infection. We found that a plasmid DNA vaccine formulated in a novel delivery system induced protective immunity against Zika virus infection in mice in a dose-dependent manner.
Hraber P, Bradfute SB, Clarke E, Ye C, and Pitard B. Amphiphilic Block Copolymer Delivery of a DNA Vaccine against Zika Virus. Vaccine 36:6911 (2018)

The Bradfute lab is involved in testing novel vaccines for induction of cross-reactive immune responses against multiple encephalitis viruses, which are mosquito-borne and cause severe disease in both horses and humans.
-- Nanocarrier antigen delivery for broadly protective, single-dose alphavirus vaccines.
We are partnering with Los Alamos National Laboratories (LANL) to test a novel DNA-based vaccine to provide single-shot, long-lasting immunity against multiple alphaviruses. Our role in this collaboration is to test DNA vaccines for their induction of antibody and T cell responses in mice, using BSL-3 alphaviruses as targets.
-- Long non-coding RNAs in Venezuelan equine encephalitis virus infection.
We have just recently been awarded a grant for a large project studying the role of long non-coding RNAs in cellular responses to Venezuelan equine encephalitis virus infection. This project will leverage our select agent BSL-3 facilities at UNM HSC to test which long non-coding RNAs are expressed after in vitro and in vivo infection of a pathogenic Venezuelan equine encephalitis virus compared to an non-pathogenic version of the same virus. In this manner, we will be able to directly compare which long non-coding RNAs are induced or suppressed in a successful cellular response versus an unsuccessful response.
Ebola virus is a deadly pathogen that has a mortality rate of over 40%. Although traditionally confined to small outbreaks in isolated villages, the virus began to spread to large cities for the first time, causing a global outbreak from 2013-2016. We are involved in efforts for vaccine and therapeutics development to this virus.
-Ebola virus vaccine and therapeutics development.
We found that glycosylation (sugar pattern) changes in the viral glycoprotein dramatically affects how these proteins induce an immune response when used as a vaccine. These changes in glycosylation occur when the vaccines are made in different cell types. Our findings were relevant, since different cells types are used for different vaccines.
-Ebola virus therapeutic and vaccine co-administration.
Our Ebola virus studies have continued with the recent funding of a second grant, in which we are studying how administering short-term therapeutics alongside long-term vaccines affects the efficacy of these treatments. The goal is to determine optimal timing for given both therapeutic drugs for immediate protection against the virus without abrogating long-term protection of co-administered vaccines

Given the urgency of the ongoing coronavirus pandemic, the Bradfute lab spearheaded the BSL-3 SARS-CoV-2 virus work at UNM HSC. We have collaborated with over 25 different academic and commercial groups to use our unique specialization to research therapeutics, vaccines, inactivation, basic biology, and patient sample analysis for SARS-CoV-2.
-- Assessment of neutralizing antibodies in convalescent and acute COVID-19 patients.
The Bradfute lab tested neutralizing antibody titers in plasma from convalescent individuals and plasma from acute COVID-19 patients that had been infused with the convalescent plasma as an experimental therapy at UNMH. We found that although all convalescent patients tested had positive antibody titers against the Spike surface protein when measure by enzyme-linked immunosorbent assays (ELISA), the levels of neutralizing antibody against live SARS-CoV-2 was very low in convalescent individuals and therefore did not boost antibody levels or ameliorate disease progressing in recipients. This study highlighted the importance of pre-screening convalescent plasma not just for total antibody via ELISA but for neutralizing antibody titers prior to infusion in patients. We have also collaborated with TriCore Reference Laboratories to show that neutralizing antibody titers correlate with a commercially used simple and rapid antibody detection assay that does not require the use of live virus.
-- Methods for inactivation of SARS-CoV-2 on surfaces.
We have tested several chemical, heat, light, and other methods to inactivate live SARS-CoV-2. We have also demonstrated that a commonly suggested method of decontaminating N95 masks with dry heat is not effective in eliminating live SARS-CoV-2.
-- Analysis of longitudinal antibody and T cell responses in convalescent COVID-19 individuals. We have recently been funded by UNM HSC CTSC to track immune responses in patients after their recovery from SARS-CoV-2 infection. We have an IRB approved for this work and are currently recruiting 50 patients to analyze their antibody and T cell responses for up to 10 years. This work will answer vital questions regarding how long COVID-19 survivors have immune responses against the virus.
-- In vitro screening of small molecules against SARS-CoV-2.
One of the major aspects of my SARS-CoV-2 work centers on testing small molecules for their in vitro efficacy against live virus in the BSL-3 laboratory. We have worked with UNM HSC, UNM main campus, and non-UNM institutions to test their drugs for antiviral activity. My lab has screened many small molecules and have found a handful that potently inhibit replication. This work has resulted in many grant submissions as well as manuscripts in preparation or submitted, a sample of which is listed below.