Principal Investigator:
Matthew Campen, PhD
Professor,
College of Pharmacy
MCampen@salud.unm.edu
(505) 272-3329
Lab Physical Address
Nursing Pharmacy Building, room B-3
Lab Mailing Address
Department of Pharmaceutical Sciences
MSC09 5360
1 University of New Mexico
Albuquerque, NM 87131-0001

Top: Jessica Begay (MS Student), Tamara Young (PhD student), Alexis Wilson (UPN student), Raul Salazar (Pharm.D. student), Katherine Zychowski, Ph.D. (Research Assistant Professor).
Bottom: Matthew Campen, Ph.D. (Professor), Guy Herbert (Research Specialist) Russell Hunter (Ph.D. student), Selita Lucas (Research Specialist), Jesse Denson, Ph.D. (Scientific Communications Director), Thomas Wilson (Pharm.D. student), Barry Bleske, Pharm.D. (Chair, Pharmacy Practice and Administrative Sciences).
Dr. Campen leads the Cardiovascular Toxicology Laboratory in the Department of Pharmaceutical Sciences. Joined by colleagues Barry Bleske, Pharm.D. and Katherine Zychowski, Ph.D., the group investigates cardiovascular impacts of environmental toxicants, primarily. Current research involves understanding how inhaled toxicants, including nanomaterials, uranium mine-derived particulates, and ozone, can negatively affect blood vessels throughout the body.
Dr. Campen directs the KL2 Mentored Career Development Program within the UNM Clinical and Translational Sciences Center. He is also Deputy Director for the UNM Metal Exposure and Toxicity Assessment on Tribal Lands in the Southwest (UNM METALS) Superfund Research Program Center. These programs emphasize the translation of environmental health and toxicology research from bench-to-bedside – or bench-to-trench, as in the case of community work – and this translational relevance is built into much of the Cardiovascular Toxicology Laboratory approach.
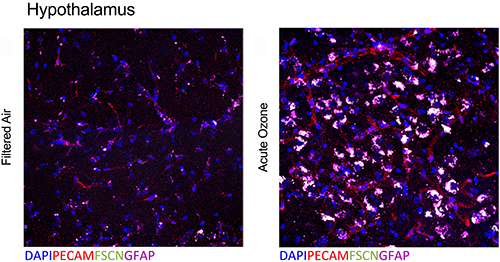
Overlapping blood–brain barrier (BBB) impairment and astrocyte activation at the neurovascular unit following acute O3 exposure (Tyler et al., Toxicol Sci, 2019).
Air pollution, especially particulate matter (PM), has a substantial impact on global health, with the World Health Organization estimating 800,000 annual excess cardiopulmonary deaths through mechanisms that are not entirely clear. Ongoing work in our program has revealed potentiating interactions between combustion- source PM and associated gaseous components that can enhance systemic vascular toxicity. Despite the protective barrier afforded by the lung, the systemic vascular endothelium is a vulnerable target of air pollution toxicity and sensitive to the combinations of PM and gas components. Classic outcomes of inflammatory endothelial activation are observable in animals and humans exposed to a wide variety of pollutants; such responses are central to early development of atherosclerosis and also to late-stage events, such as plaque instability and rupture. What remains unclear is the pathway by which air pollution toxicity is transferred from the lung to the vasculature. We postulate that inflammatory endothelial activation arises following inhalation of air pollutants due to oxidative modifications of blood components, and reflects a common mode of action for many pollutants. Plasma obtained from humans exposed to diesel or nitrogen dioxide activates endothelial cells, suggesting that the transfer of toxicity from the lung to systemic vessels is carried in the blood stream. Multiligand scavenger and pattern recognition receptors on endothelial cells, including the lechtin-like receptor for oxidized LDL (LOX-1) and CD36, may represent a focal junction that reduces the complex serum alterations to common vascular pathological responses. The objectives of the renewed project are to expand on two key findings from the original project: 1) gases and PM interact to enhance vascular toxicity and 2) inhaled pollutants increase circulating inflammatory potential. Thus in Aim 1, we will elucidate interactions between PM and volatile organics in driving systemic vascular toxicity. Here, we hypothesize that combining PM with the gaseous portion of motor vehicle emissions will enhance vascular toxicity in a manner dependent on the surface area and composition of the PM. In Aim 2, we will measure circulating inflammatory potential relative to components of combined engine emissions, and analyze results using novel statistical methods designed for complex mixtures. We hypothesize that the potency of acute endothelial cell activation by circulating factors will be dose-dependent, exacerbated by combined PM and gas phases, and will correlate with chronic vascular remodeling and oxidative stress. Lastly in Aim 3, we will delineate the relative contribution of CD36 and LOX-1 in driving pulmonary generation of and endothelial response to circulating factors induced by O3 and MVE. We hypothesize that despite the complex atmospheric chemistry and serum composition changes with pollution exposures, the multiligand receptors CD36 and LOX-1 mediate endothelial response and NOS inactivation. Successful completion of these Aims will identify new pathways that mediate transference of air pollution toxicity to systemic vasculature that contributes to cardiovascular morbidity.
(with Dr. Andrew Ottens, below):
Engineered nanomaterials (ENMs) have an unknown toxic potential and the relationship between the biological effects and the physicochemical properties remains uncertain. Our long-term research goal is to enable an efficient and accurate safety profiling of ENM of varying characteristics and conditions of exposure that would enhance decision-making regarding human exposure risks. Our objective in this application is to determine serum compositional changes resulting from pulmonary ENM exposure that lead to systemic toxicity (vascular and neural, primarily). We propose the following specific aims, which will pair innovative ex vivo bioactivity outputs with advanced high-resolution mass spec compositional analysis. In the first Aim, we will ascertain mechanisms of pulmonary origin of modified circulatory components related to metalloproteinase activity and inflammation. Evidence suggests that circulating factors may be related to a) direct reactions of ENMs and pulmonary cellular/molecular components or b) degradation by-products of increased metalloproteinase activity and that the circulating components act through endothelial cell surface pattern recognition receptors. In the second Aim, we will assess neurovascular and central nervous system impacts arising from pulmonary ENM- induced serome modifications. Here, we will develop and optimize a sequence tag-based algorithm to enhance the identification of endogenous peptides within the multi-walled carbon nanotube (MWCNT)-responsive serome, which will facilitate evaluation of the ENM-induced bioactivation in the cerebrovasculature and the diffusivity of ENM-responsive serome factors across the blood brain barrier. Lastly, in the third Aim, we will assess MWCNT-associated circulatory characteristics and inflammatory potential in samples derived from an occupationally-exposed cohort. We will assess the serum bioactivity in terms of endothelial activation and link to personal breathing zone measurements of elemental carbon, a marker of carbon nanotube/nanofiber exposure. At the completion of this project, we expect to have identified key physiological and chemical factors that influence plasma-borne ENM toxicity. The successful completion of these studies is expected to constitute an important step towards preventing / attenuating adverse health effects associated with ENM exposure.
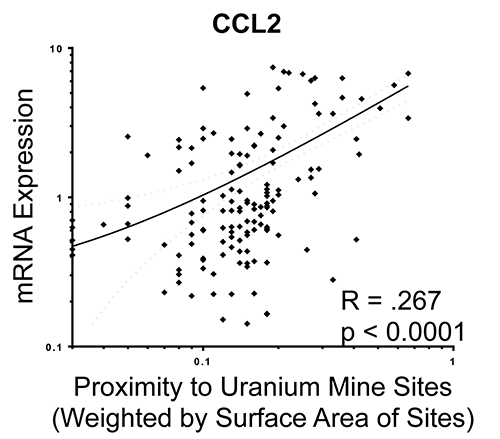
Linear regression plot of weighted abandoned uranium mine proximity associations with chemokine ligand 2 (CCL2) mRNA from endothelial cell responses to participant serum (Harmon et al., JESEE, 2017).
Cardiovascular and metabolic diseases are on the rise nationally and a growing body of evidence highlights a role for environmental contaminants as adjunctive cardiovascular disease (CVD) factors. In New Mexico and the Navajo Nation, many abandoned and unremediated mining regions exist and continue to contaminate the land, water, and air. Inhalation of metal-rich particulate matter (PM) from mining waste may pose an unrecognized risk for cardiovascular and pulmonary disease in affected communities. A strong link exists between metals in airborne PM and adverse cardiovascular and pulmonary outcomes, especially chronic inflammatory vascular disease. However, much of the toxicology has focused on soluble forms of metal, which are more relevant to the burning of residual oil in the shipping industry. There are numerous sites in the Southwest US where mining waste has led to severe soil contamination of metals mixtures, leading to high levels of uranium (U), copper (Cu), vanadium (V), nickel (Ni), and arsenic (As), among others. We will assess direct and indirect atherogenic impacts of inhaled particulate matter obtained from communities with a history of mixed metals contamination. The working model relates to complex interactions in the lung that lead to secondary circulatory products that induce vascular endothelial inflammatory responses. Immunomodulatory receptors, such as CD36, TLR4, and the lechtin-like receptor for oxidized low-density lipoprotein (LOX-1) mediate vascular responses to other solid and gaseous components of air pollution. The impact of metals in driving vascular innate immune responses is poorly understood. We hypothesize that pulmonary exposures to metal-rich PM from mine waste-contaminated tribal regions will generate circulating factors such as oxidized LDL, which in turn activate inflammatory response and dysfunction in endothelial cells, dependent on immunomodulatory receptors. The following Aims will address this hypothesis in a mechanistic and translational manner. In the first Aim, we will compare the potency of inhaled dust samples from mining regions in terms of driving systemic vascular toxicity and serum inflammatory potential. In the second Aim, we will examine the role of oxLDL and immunomodulatory receptors in driving endothelial activation and dysfunction stemming from exposure to metal-rich PM. We will selectively antagonize the oxLDL/LOX-1 pathway in vivo and in vitro to assess outcomes of endothelial dysfunction and vascular inflammation. Lastly, in the third Aim we will model downwind exposures in a Navajo cohort to examine associations with circulating markers of endothelial injury and serum inflammatory potential. We will model windblown dust exposures and link to outcomes of inflammatory / endothelial injury markers (oxLDL, soluble ICAM and VCAM, endothelin-1) and serum bioactivity from a cohort of 252 members of the Navajo Nation. Characterization of health impacts of repairable PM from contaminated sites will provide important information related to hazard identification and biological plausibility to support prioritization of risk management and remediation strategies.
Tyler CR, Zychowski KE, Sanchez BN, Rivero V, Lucas S, Herbert G, Liu J, Irshad H, McDonald JD, Bleske BE, Campen MJ. Surface area-dependence of gas-particle interactions influences pulmonary and neuroinflammatory outcomes. Part Fibre Toxicol. 13:64, 2016. PMID: 27906023; PMC5131556
Zychowski KE, Sanchez B, Pedrosa RP, Lorenzi-Filho G, Drager LF, Polotsky VY, Campen MJ. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis. 254:59-66, 2016. PMID: 27693879; PMC5097675
Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Pacheco B, Erdei E, Ramone S, Nez T, Gonzales M, Campen MJ. Residential Proximity to Abandoned Uranium Mines and Serum Inflammatory Potential in Chronically Exposed Navajo Communities. J Exposure Sci Environ Epidemiol.27:365-371, 2017. PMID: 28120833; PMC5781233
Aragon M, Topper L, Tyler CR, Sanchez BN, Zychowski KE, Young T, Herbert G, Hall P, Erdely A, Eye T, Zeidler-Erdely P, Ottens AK, Campen MJ. Serum-Borne Bioactivity Caused by Pulmonary Multiwalled Carbon Nanotube Exposure Induces Neuroinflammation Via Blood Brain Barrier Impairment. Proc Natl Acad Sci USA, 114:E1968-E1976, 2017. PMID: 28223486; PMC5347541
Zychowski KE, Kodali V, Harmon M, Tyler CR, Sanchez B, Ordonez Suarez Y, Herbert G, Wheeler A, Avasarala S, Cerrato JM, Kunda NK, Muttil P, Shuey C, Brearley A, Ali AM, Lin Y, Shoeb M, Erdely A, Campen MJ. Respirable Uranyl-Vanadate-Containing Particulate Matter Derived From a Legacy Uranium Mine Site Exhibits Potentiated Cardiopulmonary Toxicity. Toxicol Sci. 164: 101-114, 2018. PMID: 29660078; PMCID: PMC6016706.
Tyler CR, Noor S, Young TL, Rivero V, Sanchez B, Lucas S, Caldwell KK, Milligan ED, Campen MJ. Aging Exacerbates Neuroinflammatory Outcomes Induced by Acute Ozone Exposure. Toxicol Sci.163:123-139, 2018. PMID: 29385576; PMC5920500
Mostovenko E, Young T, Muldoon PP, Bishop L, Canal CG, Vucetic A, Zeidler-Erdely PC, Erdely A, Campen MJ, Ottens AK. Nanoparticle exposure driven circulating bioactive peptidome causes systemic inflammation and vascular dysfunction. Part Fibre Toxicol. 16:20, 2019. PMID: 31142334; PMC in progress.
Matthew Campen, PhD
Professor, Principal Investigator,
UNM College of Pharmacy
Physical Address
Nursing/Pharmacy Building
2502 Marble Ave. NE
Suite B-3
Albuquerque, NM 87131-0001
Mailing Address
Department of Pharmaceutical Sciences
MSC09 5360
1 University of New Mexico
Albuquerque, NM 87131-0001