The IMAC is housed on the ground floor of the College of Pharmacy and provides the UNM Center for Metals in Biology and Medicine investigators and members with the necessary expertise and tools for a variety of modern molecular analyses, including ICP-MS, EPR spin-trapping, and quantitative mass spectrometry.
Beyond providing access to the advanced analytical instrumentation present in facility, the Core provides in-house analyses, supports innovative protocol development, and provides training to students, fellows, and faculty in the integrated applications associated with the instrumentation. The IMAC is open to all scientists interested in using novel instrumentation to investigate environmental health-related questions.

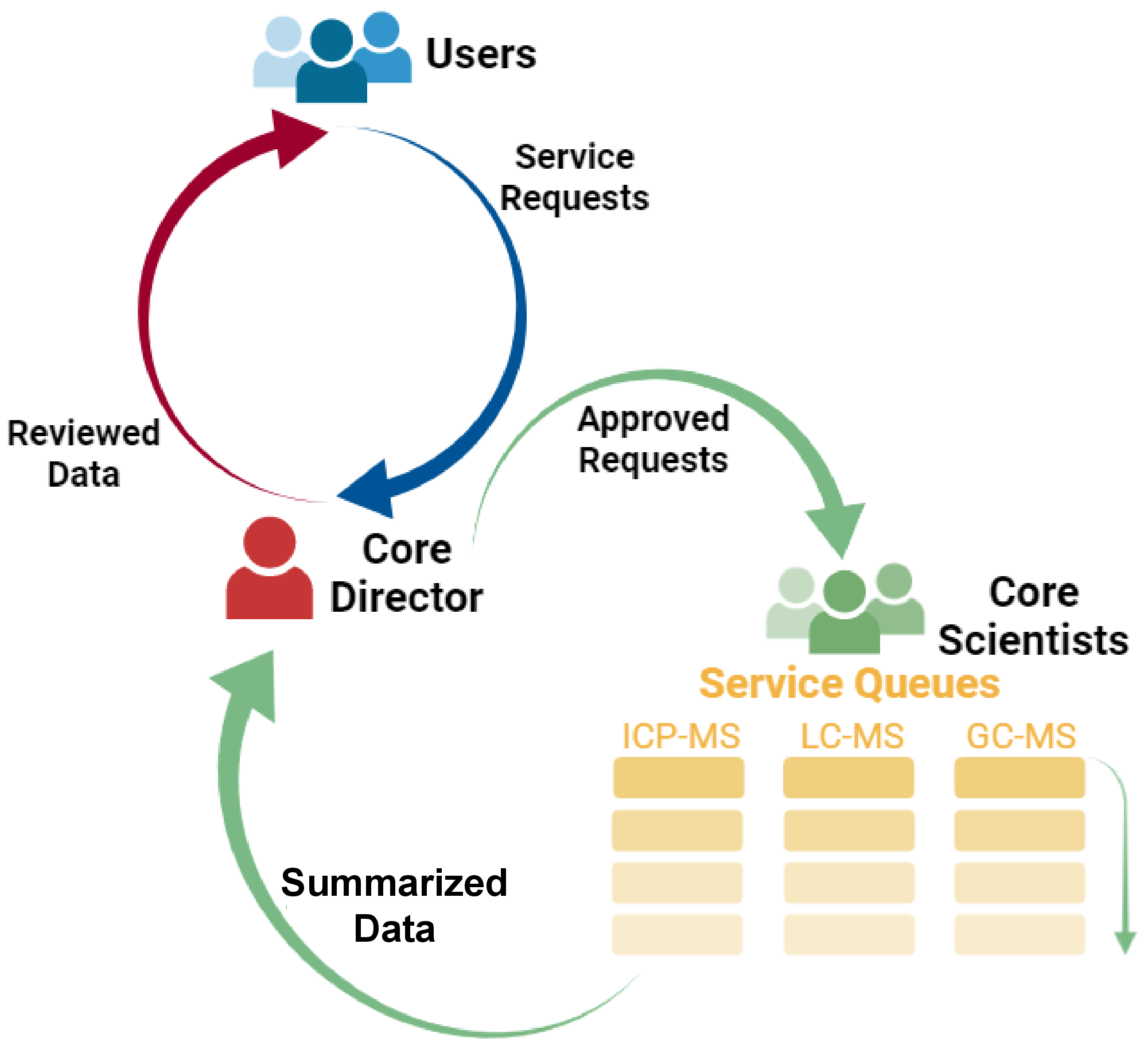
To oversee the efforts and resources toward services and research, the Core operates in a formalized management fashion (as shown on the scheme below and on our Standard Operational Procedure). Users are required to sign the User Agreement Form before submitting any samples to the Core. The Core can decline certain service request if not feasible, or request the prospective users to revise their requests. If the request is deemed feasible, the Core Director will assign the request to the corresponding Core scientist for him/her to generate and distribute a quote on the projected costs.
Once the user accepts the quote, the Core scientist will add the request to the queue for processing and testing, based on the priority level determined by the Core, and schedule the sample delivery by the user. The Core employs the sample queue workflow: up to 5 approved requests will be in each of the current testing queues per instrument, and new samples will not be accepted until a vacancy emerges. This is to ensure that (a) the shared resource will be utilized effectively, (b) the analysis will be completed on time, and (c) there is a clearer expectation in terms of the testing completion timeline. The Core will communicate with the user about the estimated schedule for the Core to carry out their service request.
After the bioanalysis is done, the data will be distributed together with any technical note.
The IMAC implements a process for review and prioritization of in-house new/tailored methods to develop. It is at the Core’s discretion to decide on the type and nature of the user’s request (i.e., standard service or research request). The user can submit the method development request using the form.
Can be coupled with bulk solution, or single-cell analysis introductory system.
This 7900 ICP-MS has unprecedented matrix tolerance, up to 11 orders-of-magnitude dynamic range, highly effective collision cell mode and high sensitivity (>109 cps/ppm at <2% CeO) yet reduced background. Its capability of fast analysis with transient signals combined with a high transport-efficiency microFAST Single Cell Automation System enables advanced analysis of trace elements in single cells and nanoparticles. An efficient conventional sample introduction system is also equipped for solution-based analysis.
Can be coupled with UltiMate 3000 RSLCnano, or Vanquish Flex Binary UHPLC.
This MS system combines high-performance quadrupole precursor selection with high-resolution, accurate-mass (140,000 resolution at m/z 200 and <1 ppm mass accuracy) Orbitrap detection for outstanding performance and tremendous versatility. It is superbly suited to untargeted or targeted screening with high-confidence confirmation, and is equally capable of a broad range of qualitative and quantitative applications. By coupling to a Vanquish UHPLC or an UltiMate 3000 RSLCnano, high throughput or high sensitivity research needs can be met.
The triple Quad LC-MS/MS system delivers superior quantitative results in a single injection workflow. Known for its accurate quantitation capabilities, the instrument enables scientists to acquire multiple reaction monitoring (MRM), precursor, neutral loss, and product ion scans to develop powerful methods for complex matrices, all while maintaining reproducibility and robustness. With the QTRAP functionality, the instrument acts as a linear ion trap (LIT). This allows the researcher to acquire even more data from the sample without sacrificing the sensitivity or quality of MRM analysis.
The spectrometer is equipped with a standard TE102 resonator, and variable temperature control system and is primarily used for the measurement of small samples, such as chemical solutions, tissue homogenates, tissue slices, and cultured cells. The Bruker ELEXSYS- II E-540 EPR spectrometer is equipped with a L-band bridge (1.0 GHz, low frequency), which allows us to acquire in vivo spectroscopy and imaging of free radical formations in small animal models such as rats and mice.
For analysis of volatiles in liquid samples.
The University of New Mexico Center for Metals in Biology and Medicine is funded by the IDeA program of the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS), along with support from the UNM College of Pharmacy, the UNM Health Sciences Center, the UNM UNM Comprehensive Cancer Center, and the UNM Clinical and Translational Science Center.
NIH NIGMS requires acknowledgment of the UNM CMBM COBRE in publications, abstracts, and presentations if the research being reported on has benefitted from the CMBM. Please acknowledge the COBRE using wording such as “Supported by UNM CMBM through NIH NIGMS grant P20 GM130422” if you (or your PI) has received COBRE funding as a Project Leader or Pilot Project PI, or if you have consulted with staff or used services/resources from the IMAC.
Please also remember to follow the NIH Public Access Policy by linking your publication from PubMed to NIH NIGMS grant P20 GM130422 and by ensuring that your manuscript is assigned a PubMed Central ID (PMCID).
Current Publications
 |
Dr. Jim Feng, IMAC Director Dr. Feng is an established researcher in biophysics, biochemistry and physical chemistry. He has 98 peer-reviewed publications and directs an active NIH- and NSF-funded research program to decipher the structure-dynamics-function relationship of multidomain enzymes. He has applied various mass spec techniques (MALDI-MS, LC-MS/MS, FT-ICR) extensively to characterizations of both metalloproteins and complex inorganic species. These studies share a common theme in that they require multidisciplinary approaches across the broad fields of mass spectrometry and spectroscopy to decipher the molecular mechanisms of metalloproteins.
|
 |
Dr. Ting Jiang, Research Assistant Professor, IMAC Science Research Manager Dr. Jiang has 10 years hands-on experience in using a wide range of mass spectrometers to solve diverse scientific problems. Specifically, she has routinely used MALDI-TOF and LC-MSD for screening and validating chromophore synthesis products, triple quadrupole LC-MS for accurate quantitation of trace-level perfluoroalkyl substances in human serum, and HRMS QTOF for uncovering emerging industrial chemicals in environmental and biological samples. Dr. Jiang is in charge of the LC MS analysis. |
|
|
Dr. Rui Liu, Research Scientist 3 Dr. Liu has 7 years firsthand experience in using ICP-MS to analyze trace metals. On top of his experience in troubleshooting and maintaining instruments, his expertise is in developing and validating new ICP-MS analytical methods. Dr. Liu is also in charge of GC-MS analysis at the Core. |
|
|
Dr. Jing Yang, Research Associate Professor, EPR Specialist Dr. Yang is an active researcher in the field of inorganic chemistry, physical chemistry, and biochemistry, with the primary interest in the metalloproteins. Her expertise includes the computational modeling and spectroscopic methods (EPR, photoluminescence, MCD, rR, and XAS). She has published 30 peer-reviewed journal articles. Dr. Yang has more than fifteen years of experience on the multifrequency EPR spectroscopy collection and spectral simulation for identifying and exploring a wide variety of EPR-active species (radicals and transition metals) found in the synthetic molecules or enzyme samples to study the relationship of geometric and electronic properties to their functions. Dr. Yang is in charge of the EPR data acquisition and analysis at the CORE. |
Hours
UNM regular business hours, 9 am – 5 pm; lunch break noon – 1 PM
Location
IMAC Core, CMBM Center
Nursing/Pharmacy Building, Building #228
2502 Marble NE, Room B54
Albuquerque, NM 87131