Ophthalmology Research
The UNM Ophthalmology Division is engaged in basic science as well as clinical research in ophthalmology and visual sciences. Our investigators aim at the pathophysiology of angiogenesis, blood-retinal barrier, diabetic retinopathy and age-related macular degeneration, as well as in photoreceptor biology. Our goal is to translate novel ideas from the laboratory to the clinics thus contributing to development of novel therapeutic strategies and biomarkers for ocular diseases.
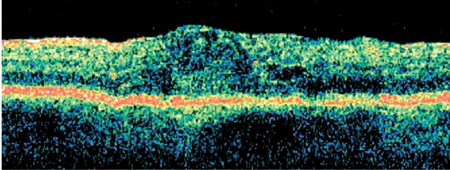
OCT images of a patient with diabetic macular edema.
We are actively involved in the mission of teaching medical students, residents, and postdoctoral fellows, and encourage the new generation to participate in vision research. We are participating in several clinical trials in ophthalmology as well as multidisciplinary landmark trials in diabetic retinopathy in collaboration with the Endocrinology and Nephrology Divisions.
Meet Our Researchers
Current Studies
All studies have been approved by the Institutional Review Board.
YOSEMITE Study
A Study to Evaluate the Efficacy and Safety of Faricimab (RO6867461) in Participants With Diabetic Macular EdemaObjective: This study will evaluate the efficacy, safety, and pharmacokinetics of faricimab administered at 8-week intervals or as specified in the protocol following treatment initiation, compared with aflibercept once every 8 weeks (Q8W), in participants with diabetic macular edema (DME).
Sponsor: Hoffmann-La Roche
Principal Investigator: Arup Das, MD, PhD
Clinical Coordinator: Hunter Esmiol
FOCUS Study
Long-Term effects of semaglutide on diabetic retinopathy in subjects with type 2 diabetes.Objective: The objective is to assess the long-term effects of treatment with semaglutide compared to placebo, both added to standard-of-care, on diabetic retinopathy development and progression in subjects with T2D.
Sponsor: Novo Nordisk
Investigator: Arup Das, MD, PhD; Primary Investigator: Mark Burge, MD
Clinical Coordinator: Hunter Esmiol
PORTAL Study
Extension Study for the Port Delivery System With Ranibizumab.Objective: This study will evaluate the long-term safety and tolerability of the Port Delivery System (PDS) with ranibizumab 100 mg/mL with refills administered every 24 weeks (Q24W) for approximately 144 weeks in participants with neovascular age-related macular degeneration (nAMD) who have completed either Phase II Study GX28228 or Phase III Study GR40548.
Sponsor: Hoffmann-La Roche
Primary Investigator: Joaquin Tosi, MD
Clinical Coordinator: Hunter Esmiol
DPPOS Study
Diabetes Prevention Program Outcomes StudyObjective: A multi-center clinical trial that examines the efficacy of an intensive lifestyle intervention or metformin to prevent or delay the development of Type 2 diabetes in a population selected to be high-risk due to the presence of impaired glucose tolerance.
Sponsor: National Institutes of Health (NIH)
Investigator: Arup Das, MD, PhD; Primary Investigator: David Schade, MD
Clinical Coordinator: Janene Canady, RN
EDIC Study
Epidemiology of Diabetes Interventions and ComplicationsObjective: This is the national long-term follow-up study of the same group studied in the Diabetes Control and Complications Trial (DCCT). The volunteers in this study have type1 diabetes. EDIC follows these participants to find out if intensive therapy provides risk reduction for retinopathy, nephropathy and neuropathy.
Sponsor: National Institutes of Health (NIH); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Principal Investigator: David Schade, MD
Investigator: Arup Das, MD, PhD
Clinical Coordinator: Janene Canady, RN
Completed Studies
BOULEVARD Study
A Study of Faricimab (RO6867461) in Participants With Center-involving Diabetic Macular Edema.
Objective: This is a multiple-center, multiple-dose, randomized, active comparator-controlled, double-masked, three parallel group, 36-week study in participants with center-involving diabetic macular edema (DME). Only one eye will be selected as the study eye. Where both eyes meet all eligibility criteria, the eye with the worse best corrected visual acuity (BCVA) will be defined as the study eye. The study will consist of a treatment period (20 weeks) and an observational period (up to 16 weeks). Treatment naive participants will be randomized in a 1:1:1 ratio to one of the Arms A, B and C, respectively. Participants previously treated with intravitreal (IVT) anti-vascular endothelial growth factor (VEGF) will be randomized in a 1:1 ratio to Arms A and C.
Sponsor: Hoffmann-La Roche
Primary Investigator: Arup Das, MD, PhD
Coordinator: Abigail Cunnigham
LADDER Study
Study of the Efficacy and Safety of the Ranibizumab Port Delivery System (RPDS) for Sustained Delivery of Ranibizumab in Participants With Subfoveal Neovascular Age-Related Macular Degeneration (AMD).
Objective: This is a Phase II clinical study to evaluate the efficacy, safety and pharmacokinetics of three different formulations of ranibizumab delivered via RPDS implant compared with the standard of care (SOC) intravitreal (ITV) injections of ranibizumab, in participants with subfoveal neovascular AMD.
Sponsor: Genentech, Inc.
Primary Investigator: Joaquin Tosi, MD
Coordinator: Abigail Cunnigham
DRCR Protocol T
A Comparative effectiveness study of intravitreal aflibercept, bevacizumab and ranibizumab for diabetic macular edema
Objective: This multi-site study compares the effectiveness of three drugs commonly used to treat the edema of the macula in the eyes of diabetic patients.
Sponsor: National Institute of Health (NIH) funding to the DRCR network, collaborative network dedicated to facilitating multicenter clinical research of diabetic retinopathy, diabetic macular edema and associated conditions.
Primary Investigator: Arup Das, MD, PhD
Coordinator: Linda Friesen
RIDE Study
A Phase III, Double-Masked, Multicenter, Randomized, Sham-Injection Controlled Study of the Efficacy and Safety of Ranibizumab (Lucentis) Injection in Subjects with Clinically Significant Macular Edema with Center involvement Secondary to Diabetes Mellitus
Objective: This clinical trial evaluated the safety and effectiveness of ranibizumab for diabetic macular edema, the major cause of significant visual loss in diabetics. Data from this and other studies was used to gain FDA approval for the use of ranibizumab in the treatment of diabetic macular edema.
Sponsor: Genentech, Inc.
Principal Investigator: Arup Das, MD, PhD
Clinical Coordinator: Sheila Nemeth, COMT; Linda Friesen
READ-2 Study
Ranibizumab (Lucentis) for Edema of the Macula in Diabetes: a Phase II Study
Objective: This is a multi-center clinical trial examined the effectiveness and safety of ranibizumab, with or without laser treatment, compared to laser-only treatment for diabetic macular edema.
Sponsor: Juvenile Diabetes Research Foundation and Genentech, Inc.
Primary Investigator: Arup Das, MD, PhD
Clinical Coordinator: Sheila Nemeth, COMT
